Representative Research Publications
Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrys 2018 > Representative Research Publications > Research Results Home
Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr
- Science Advances / 2018 June
- Ryu, Kyoung Seok (Corresponding author)
Study Summary
Quorum sensing (QS), a bacterial process that regulates population-scale behavior, is mediated by small signaling molecules, called autoinducers (AIs), that are secreted and perceived, modulating a “collective” phenotype. Because the autoinducer AI-2 is secreted by a wide variety of bacterial species, its “perception” cues bacterial behavior. This response is mediated by the lsr (LuxS-regulated) operon that includes the AI-2 transporter LsrACDB and the kinase LsrK. We report that HPr, a phosphocarrier protein central to the sugar phosphotransferase system of Escherichia coli, copurifies with LsrK. Cocrystal structures of an LsrK/HPr complex were determined, and the effects of HPr and phosphorylated HPr on LsrK activity were assessed. LsrK activity is inhibited when bound to HPr, revealing new linkages between QS activity and sugar metabolism. These findings help shed new light on the abilities of bacteria to rapidly respond to changing nutrient levels at the population scale. They also suggest new means of manipulating QS activity among bacteria and within various niches.
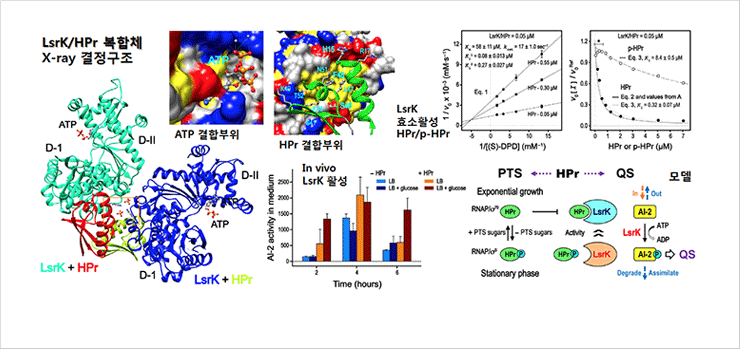 Figures. Regulation of the LsrK acivity by the ratio of HPr and p-HPr during the AI-2 mediated QS.
Figures. Regulation of the LsrK acivity by the ratio of HPr and p-HPr during the AI-2 mediated QS.
At low cell density, secreted AIs diffuse away and otherwise do not accumulate. Rather, as cells grow and continuously secrete AIs, the accumulative production of AIs at high density initiates their detection and consecutive responses. Individual cell behaviors, in turn, become properties of the collective. This transition, called QS, enables persistence in biofilms andmany other phenotypes. Abstractions of the signal transduction mechanisms have led to the design and implementation of many application-specific functions. The synchronous responses of bacteria at high density therefore provide great benefits for concerted QS signaling. QS is generally thought to be under control of transcriptional regulation, exemplified by bacteria-specific QS phenomena such as bioluminescence, biofilm formation, and virulence factor secretion. The inhibition of LsrK by HPr discovered here provides another regulatory mechanism of QS. It is particularly noteworthy that regulation of enzyme activities typically occurs at much faster characteristic times than regulation of activities involving gene expression. This will have ramifications on the design of new synthetic biology constructs. We depict a simplified view of the regulatory structure of LsrK and HPr and their involvement in PTS and QS. That is, the phosphorylation status of HPr enables the QS of bacteria to be directly linked to their metabolic state; the previous report showing the involvement of ptsI in the import of AI-2 and the transcription of lsr operon can now be explained by the direct interaction of HPr and p-HPr with the LsrK protein. The de novo regulation of LsrK activity by HPr and p-HPr that uses a protein-protein interaction and, at the same time, enables self-identification of a bacterium’s metabolic state could provide additional ways to synchronize the responses of bacteria to AI-2 and, more importantly, to differentiate the QS of certain bacteria in mixed microbial flora according to their metabolic states.



