Representative Research Publications
2017 > Representative Research Publications > Research Results Home
Synthesis and Structural Analysis of Co(IV)-Oxo Complexes
- Nature Communications / 2017 March
- Kim, Sun Hee (corresponding author)
Study Summary
Elucidation of the structure and reactivity of cobalt-oxo complexes that act as short-lived reaction intermediates with high-valent oxidation numbers.
Metal-oxo species have been extensively studied because they are important intermediates in reactions of metalloenzymes and biomimetic complexes mimicking such enzymes, especially in oxidizing reactions of water and organic substrates. However, since metal-oxo species of late transition metals (e.g. Co, Ni, or Cu), which possess many electrons in d-orbitals, are very unstable, it was previously considered to be very difficult to produce complexes mimicking such species. The present study analyzed cobalt-oxo species using various spectroscopic methods, and investigated their reactivity.
In the present study, a CW/Pulse EPR system available at the Korea Basic Science Institute (Western Seoul Center) was used to directly observe changes of the oxidation state of metal-oxo species in the catalytic reaction.
Expected Effects
The present study produced late transition metal-oxo species although such production was previously considered to be very difficult. In particular, cobalt-oxo species successfully produced in the present study have been speculated to be important intermediates in water oxidizing reactions, and this study suggested direct evidence in favor of such speculation. Thus, the present study showed the potential of cobalt-oxo species as intermediates in many difficult reactions, especially in water oxidation reactions.
Related Figures
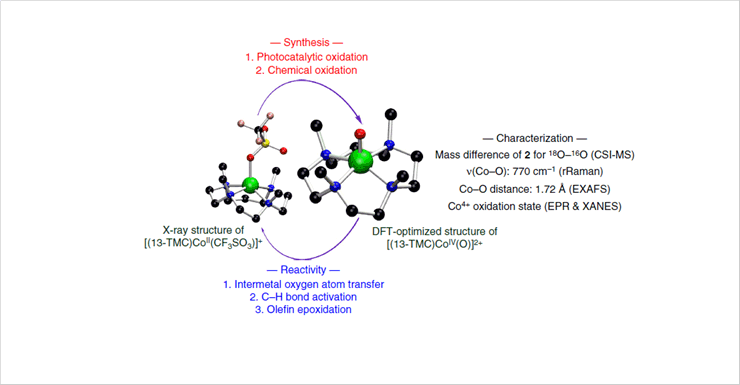 Figure 1. Synthesis, Structural Analysis, and Reactivity Investigation of Co(IV)-Oxo Species
Figure 1. Synthesis, Structural Analysis, and Reactivity Investigation of Co(IV)-Oxo Species



