Representative Research Publications
Hierarchically three-dimensional (3D) nanotubular sea urchin-shaped iron oxide and its application i 2018 > Representative Research Publications > Research Results Home
Hierarchically three-dimensional (3D) nanotubular sea urchin-shaped iron oxide and its application in heavy metal removal and solar-induced photocatalytic degradation
- Journal of Hazardous Materials / 2018 July
- Yesul Jeong(1st author), Hyun Uk Lee, Gaehang Lee(Corresponding author)
Study Summary
In this study, hierarchically three-dimensional (3D) nanotubular sea urchin-shaped iron oxide nanostructures (3D-Fe2O3) were synthesized by a facile and rapid ultrasound irradiation method. Additives, templates, inert gas atmosphere, pH regulation, and other complicated procedures were not required. Dense 3D-Fe2O3 with a relatively large Brunauer–Emmett–Teller (BET) surface area of 129.4m2/g was synthesized within 23 min, and the BET surface area was further improved to 282.7m2/g by a post heat-treatment process. In addition, this post processing led to phase changes from maghemite (γ phase) to hematite (α phase) Fe2O3. Subsequent characterization suggested that the growth mechanism of the 3D-Fe2O3 follows self-assembly and oriented attachment. The prepared 3D-Fe2O3 was applied to wastewater purification. Ultrasound-irradiated 3D-Fe2O3 can eliminate a As(V) and Cr(VI) from water with 25 times faster removal rate by using a one third smaller amount than commercial α-Fe2O3. This was attributed to the inter-particle pores and relatively positively charged surface of the nanostructure. In addition, post heat treatment on ultrasound-irradiated 3D-Fe2O3 significantly influenced the photocatalytic degradation of methylene blue and phenol, with a 25 times higher removal efficiency than that of commercial α-Fe2O3, because of both high BET surface area and good crystallization of the prepared samples.
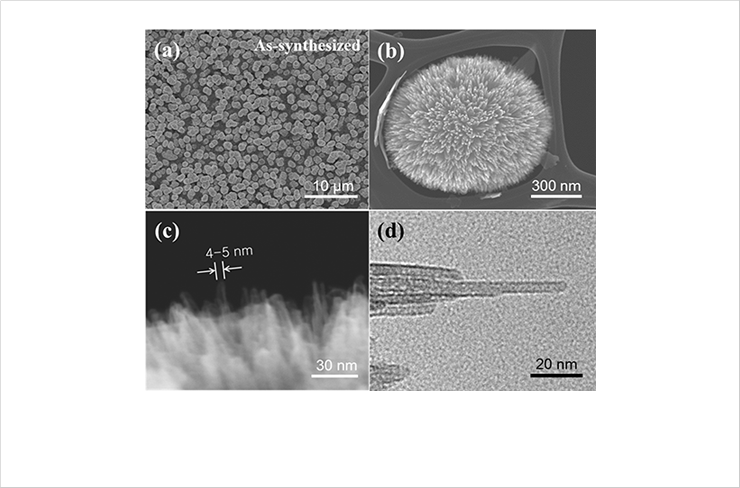 Fig. 1. SEM images of nanotubular sea urchinshaped iron oxides synthesized by ultrasound irradiation: (a) low magnification; (b) high magnification. UHR-FESEM images of nanotubular structure in nanotubular sea urchinshaped
iron oxides synthesized by ultrasound irradiation: (c) overall view; (d) single nanotubular structure
Fig. 1. SEM images of nanotubular sea urchinshaped iron oxides synthesized by ultrasound irradiation: (a) low magnification; (b) high magnification. UHR-FESEM images of nanotubular structure in nanotubular sea urchinshaped
iron oxides synthesized by ultrasound irradiation: (c) overall view; (d) single nanotubular structure
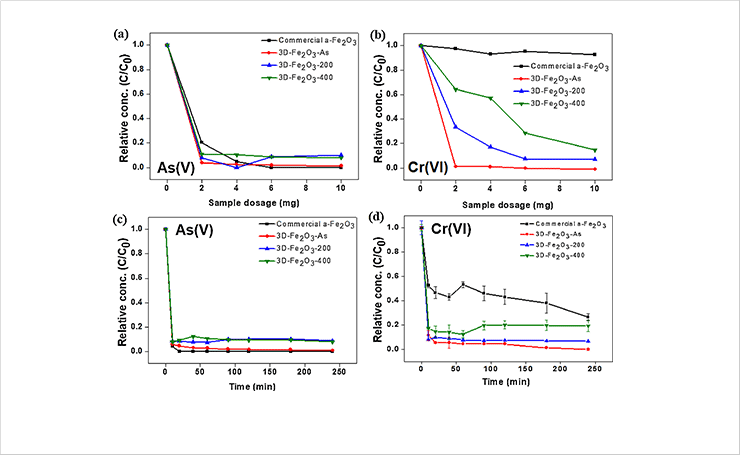 Fig. 2. Change in adsorption rate for (a, c) As(V), (b, d) Cr(VI) ions as a function of the adsorbent dosage and exposure time
Fig. 2. Change in adsorption rate for (a, c) As(V), (b, d) Cr(VI) ions as a function of the adsorbent dosage and exposure time
 Fig. 3. Photocatalytic degradation and degradation rate constants of commercial α-Fe2O3, 3D-Fe2O3-AS, 3D-Fe2O3-200, and 3D-Fe2O3-400 for (a, b) methyleneblue (MB) and (c, d) phenol.
Fig. 3. Photocatalytic degradation and degradation rate constants of commercial α-Fe2O3, 3D-Fe2O3-AS, 3D-Fe2O3-200, and 3D-Fe2O3-400 for (a, b) methyleneblue (MB) and (c, d) phenol.



