Research Center for Materials Analysis
Research Center for Materials Analysis Analytical Science > Major Research Activities Home
We focus on development and application of new analytical techniques essential for the functional improvements of materials, especially in energy/environmental sciences, and on development of related materials. This research activity is expected to lead to core knowledge and techniques for the new generation of the functional materials.
Main Research Field
Development of in-situ/operando measurement platform for energy materials
- Real-time measurements of Li-ion battery, solar cell, and fuel cell materials under operation conditions for the discovery of new energy materials
- Investigation of basic properties and growth mechanism of low-dimensional nanomaterials using Advanced In-situ surface analysis system (AISAS)
Development and application of analytical techniques to probe local dynamics of ions and molecules
- Investigation of ionic dynamics in energy conversion systems such as batteries and fuel cells
- Investigation for dynamics of water and ions dynamics in polymer electrolyte membranes
Development of energy·environment convergence materials
- Taking advantage of advanced analytical techniques, developing energy convergence materials such as lithium secondary batteries and hydrogen storage materials, and convergence materials for removing toxic materials in environment.
Development of biological convergence materials
- Development of bio-materials and analytical technologies to solve biological disasters recognized as national threatening issue.
- Development of natural compound-derived pharmaceutical materials to improve the public health.
Representative Research Cases
Research on strain relaxation of graphene layers by Cu surface roughening
- After graphene growth, the compressive strain on graphene induced by the mismatch of thermal expansion coefficient with Cu step bunching that depending on graphene layers. We found the strain relaxation of graphene layers by Cu step bunching formation using Raman spectroscopy. This finding will provide a crucial idea to enhance the electrical performance of graphene electrodes by controlling the ripple density of graphene.
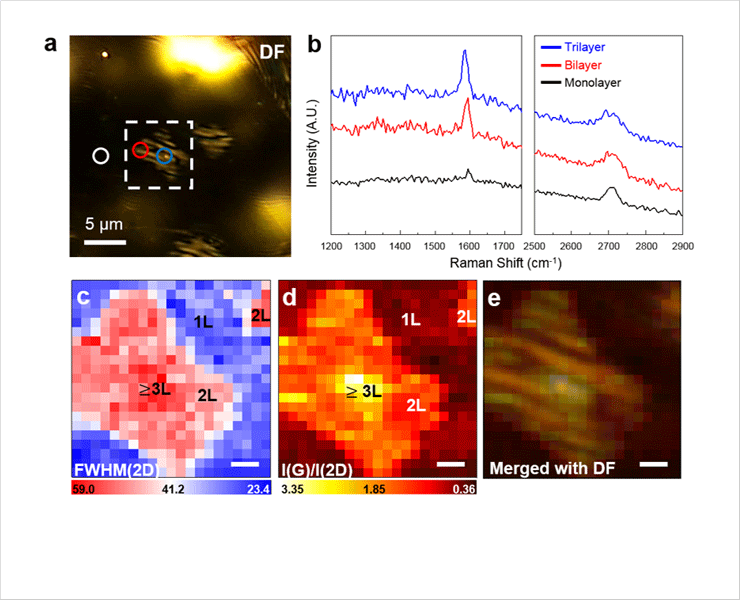 Optical microscope image and Raman spectroscopy results of the graphene on the reconstructed Cu surface. (a) optical microscope image, (b) Raman spectra, spatially resolved Raman mapping plotted with (c) FWHM(2D) and (d) intensity ratio I(G)/I(2D), (e) Dark field optical microscope image overlapped with (d).
Optical microscope image and Raman spectroscopy results of the graphene on the reconstructed Cu surface. (a) optical microscope image, (b) Raman spectra, spatially resolved Raman mapping plotted with (c) FWHM(2D) and (d) intensity ratio I(G)/I(2D), (e) Dark field optical microscope image overlapped with (d).
The first direct observation of Dirac topological states with broadband high-resolution NMR in Bi2Te3 nanoplatelets
- By combining advanced broadband solid-state NMR methods with Cs-corrected STEM and DFT calculations on pristine Bi2Te3 nanoplatelets, we succeeded for the first time to resolve the NMR signal assigned to the Dirac electron states, and showed how the Dirac electrons spread inside the nanoplatelets
-
![[Fig. 1] Band structure analysis and Dirac states of stoichiometric Bi<sub>2</sub>Te<sub>3</sub> nanoplatelets
Nat. Commun. 11, 1285 (2020)](/images/kor/sub02/rndnew_0104_2.gif) [Fig. 1] Band structure analysis and Dirac states of stoichiometric Bi2Te3 nanoplatelets
[Fig. 1] Band structure analysis and Dirac states of stoichiometric Bi2Te3 nanoplatelets
Nat. Commun. 11, 1285 (2020) -
![[Fig. 2]<sup>125</sup>Te aMAT NMR and T’2 dephasing analysis of the Dirac edge states](/images/kor/sub02/rndnew_0104_3.gif) [Fig. 2] 125Te aMAT NMR and T’2 dephasing analysis of the Dirac edge states
[Fig. 2] 125Te aMAT NMR and T’2 dephasing analysis of the Dirac edge states
Nat. Commun. 11, 1285 (2020)
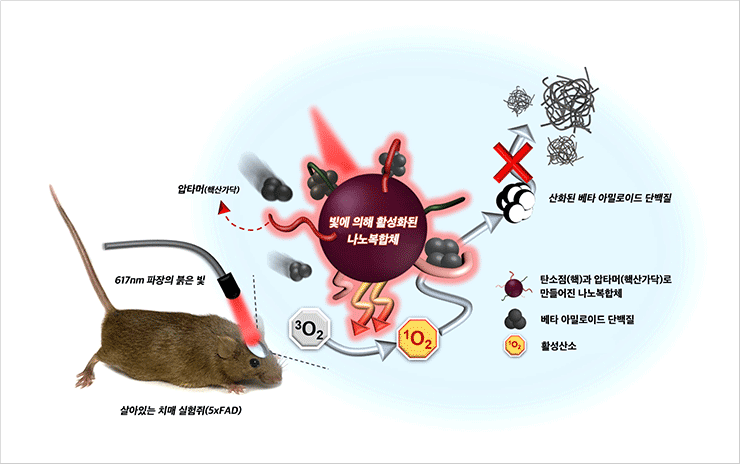
Photomodulating nanomaterial for the suppression of Alzheimer’s β-amyloid aggregation
- Photomodulating nanomaterial (Apta@CDs) present the therapeutic capabilities for light-driven suppression of Alzheimer’s β-amyloid(Aβ) aggregation.
- Apta@CDs-mediated photomodualtion modality achieves effective suppression of Aβ aggregation in vivo, which significantly reduced the Aβ burden in the 5xFAD mouse brain by ∼40%. (ACS Nano, 2020, 14, 169733)