Research Center for Bioconvergence Analysis
Research Center for Bioconvergence Analysis Analytical Science > Major Research Activities Home
The Research Center for Bioconvergence Analysis is devoted to the development and application of biotechnology with the ultimate goal of discovering how biological systems operate. Research efforts are focused on the establishment of integrated analytical platforms and the support of collaborators involved in joint research in the field of disease diagnosis and therapy. A significant contribution has been made to solving problems which are significant in the nation and in society.
Main Research Field
Biomedical omics research:
Development of new bio-omics analysis technology using analytical chemistry and bioinformatics based on mass spectrometry and result in elucidation of molecular mechanisms for life phenomena.
- Development of proteome analytical method based on high resolution mass spectrometry
- Research of the most advanced analytical technology for protein and post-translational modification of protein
- Clinical research for discovery of protein biomarkers from target disease
Study on the three-dimensional structure of proteins
We investigate the three-dimensional structure and physicochemical properties of proteins essential for life
- Study on protein structures using the state-of-the-art methodologies including high magnetic field NMR spectroscopy, X-ray crystallography, etc
- Study on the structure-based inhibitor development for protein-protein interactions
- Study on the three-dimensional structure of disease-related proteins including neurodegenerative disorders
Development of bioimaging based disease diagnosis/therapy technology
- Development and application of bio-nano material-based drug delivery system
- Development of bio-nano material (exosome)-based drug analysis platform for refractory cancer immuno-therapeutics
- Development of analysis technology for translational research based on in vitro/in vivo bioimaging
Development of rapid and sensitive diagnostic methods for infectious diseases
- Identification of diagnostic antigens and development of detecting antibodies
- Development of a rapid diagnostic test for point-of-care
- Development of an ultra-sensitive biosensor for early diagnosis
Representative Research Cases
Construction of platform for glycoproteome analysis based on mass spectrometry
- Glycoproteome analysis platform based on mass spectrometer technology was developed. Our group was participated in the International Glycoprotein Analysis (HGI) Consortium to verify the analytical method, and published a thesis. Nat. Methods, 2021, 18, p1304 (JCR 1.3%, IF 28.547, co-author)
- Using glycoproteome analysis technology, fucosylated AFP was discovered and verified as a biomarker for liver cancer diagnosis. Proteomics Clin. Appl. 2021;2000096. (JCR 38.96%, IF 2.489, principal/corresponding author)
- Registration of patent for bioinformatics processing analysis technology for identification and quantification of O-linked glycopeptides (US, 11/181,531, 2021.09.09.)
 The research results of participation in the International Glycoprotein Analysis (HGI) Consortium using glycoproteome analysis platform (left). The discovery and verification of fucosylated AFP biomarker for liver cancer diagnosis (right).
The research results of participation in the International Glycoprotein Analysis (HGI) Consortium using glycoproteome analysis platform (left). The discovery and verification of fucosylated AFP biomarker for liver cancer diagnosis (right).
Impact of Sphingosine and Acetylsphingosines on the Aggregation and Toxicity of Metal-free and Metal-treated Amyloid-β
- We revealed the mechanism of amyloid formation of metal-free and bound amyloid-β b spingosine in cerebrospinal fluid. Sphingosine and its derivative interact with amyloid-β and increase its amyloidgenesis and cytotoxicity. A new NMR method which quantifies the amount of monomers in aggregates was applied and established (Chemical Science 2021, 12, 2456-2466)
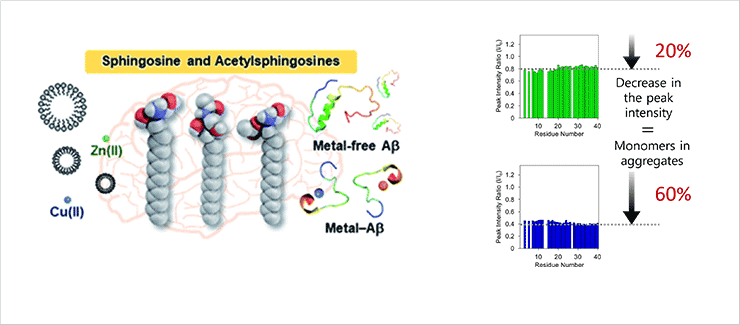 AD-causing Aβ aggregation mechanism by sphingosine(left) / NMR-based new assay(right)
AD-causing Aβ aggregation mechanism by sphingosine(left) / NMR-based new assay(right)
Development of ADC (antibody-drug conjugate) based prodrug anticancer therapeutic technology
- The near-infrared fluorescent probe was designed to selectively target tumor tissue, and the fluorescence signal was activated with high sensitivity in hypoxia in tumor tissue, suggesting its applicability as an accurate cancer diagnosis and surgical resection treatment technology (J Med Chem 2021;264:2971-2891)
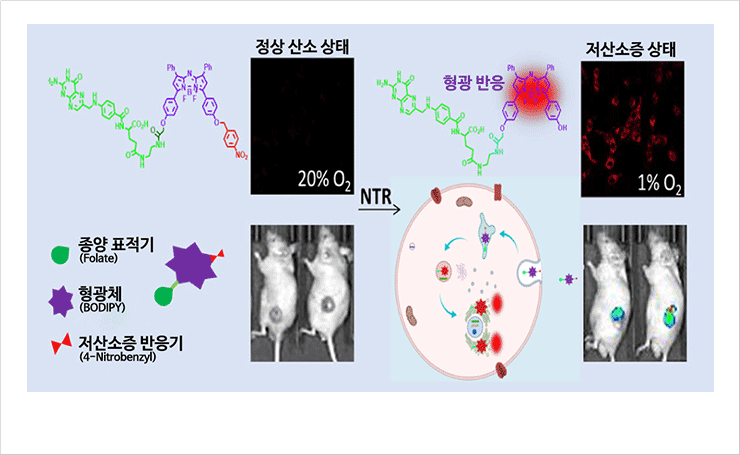 Schematic illustration of the near-infrared fluorescent probe for cancer diagnosis
Schematic illustration of the near-infrared fluorescent probe for cancer diagnosis
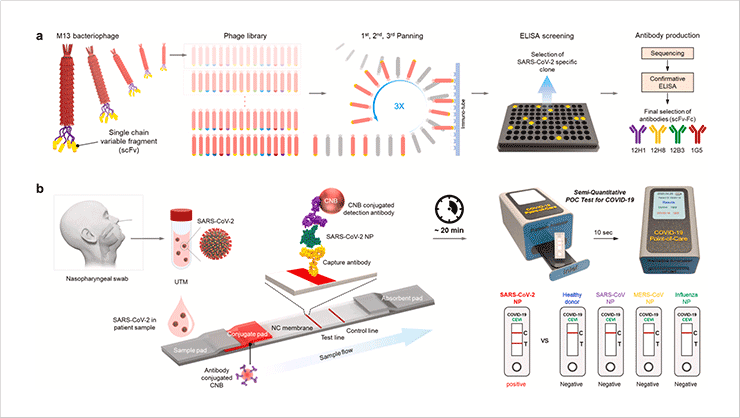
Development of rapid and sensitive diagnostic methods for infectious diseases
- We developed SARS-CoV-2 NP-specific scFv-Fc antibodies by using phage display technology. The scFv-Fc antibodies bind specifically and with high affinity to the SARS-CoV-2 NP antigen, but not to NPs of other viruses. Using these scFv-Fc antibodies, we generated a rapid diagnostic test and the diagnostic kit showed great diagnostic performance in sensitivity and selectivity.